[ Instrument Network Instrument R & D ] Under the strategic background of accelerating the transformation of energy use from fossil energy to clean energy, lithium-ion batteries (LIB) rely on their high energy density, high power, long cycle life, high operating voltage, and stable discharge The comprehensive advantages of wide operating temperature range, no memory effect, and good safety performance are particularly important in achieving environmentally friendly and efficient energy storage and conversion methods. As an important part of lithium-ion batteries, the performance of the anode itself directly affects the performance of the entire battery system.
Lithium-ion batteries use a carbon material as the negative electrode and a lithium-containing compound as the positive electrode. There is no metal lithium, only lithium ions. This is a lithium-ion battery. Lithium-ion batteries are a general term for batteries that use lithium-ion intercalation compounds as the cathode material. The charging and discharging process of a lithium-ion battery is the process of intercalation and deintercalation of lithium ions. In the process of lithium ion intercalation and deintercalation, it is accompanied by the intercalation and deintercalation of equivalent electrons such as lithium ions. During the charge and discharge process, lithium ions intercalate / deintercalate and insert / deintercalate between the positive and negative electrodes, and are known as "rocking chair batteries".
When the battery is charged, lithium ions are generated on the positive electrode of the battery, and the generated lithium ions move to the negative electrode through the electrolyte. The carbon of the negative electrode has a layered structure, and it has many micropores. The lithium ions that reach the negative electrode are embedded in the micropores of the carbon layer. The more lithium ions that are embedded, the higher the charging capacity. Similarly, when the battery is discharged (that is, the process in which we use the battery), the lithium ions embedded in the carbon layer of the negative electrode come out and move back to the positive electrode. The more lithium ions returned to the positive electrode, the higher the discharge capacity.
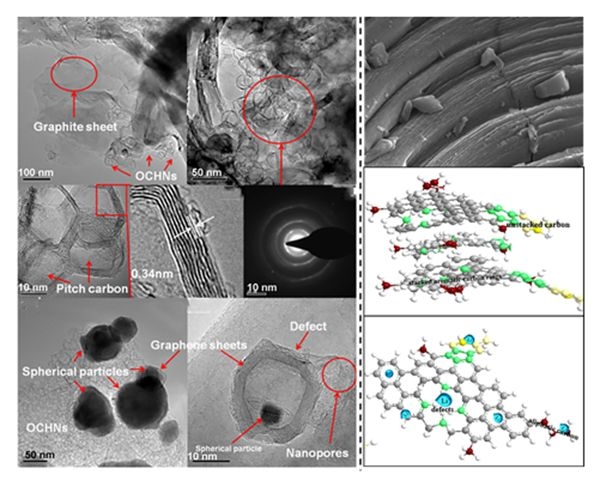
In recent years, Song Yan, a researcher at the Institute of Coal Chemistry of the Chinese Academy of Sciences, and his research team led the effective design of a series of electrode materials through structural design of carbon-based and silicon-based anode materials, achieving material specific capacity and cycle stability. And magnification performance. Based on the limitations of commercial anode material graphite in terms of structure and capacity, the team made various attempts. A nickel-doped hollow nano-carbon anode material in which graphite carbon and porous nano-carbon coexisted was prepared by hot-pressing sintering using natural graphite flakes and pitch coke as raw materials. Subsequently, the team prepared graphite-like lamellar carbon using asphalt as the raw material through compression and polymerization. This material not only has the strong stability of graphite when it is used as a negative electrode material, but its capacity value has also been improved. In view of the poor cycling stability of silicon-based anode materials, the team used electrostatic interaction to adsorb cationic surfactants on the surface of silicon nanoparticles to achieve double-layer protection of the core and shell, reducing and limiting the damage to the material structure caused by stress during silicon expansion. In order to further regulate the performance of the silicon-based double-clad structure, a hard template method is used to introduce a cavity to mitigate the volume change of silicon, and achieve the double-layer goals of increasing capacity and cyclic stability.
Graphite is a transitional crystal between atomic crystals, metal crystals and molecular crystals. In the crystal, carbon atoms in the same layer are sp2 hybridized to form a covalent bond. Each carbon atom is connected to another three carbon atoms. Six carbon atoms form a regular hexagonal ring on the same plane and stretch to form a sheet structure. . Carbon atoms in the same plane each have one p orbital, which overlap each other, and the delocalized π-bond electrons can move freely in the crystal lattice and can be excited, so graphite has a metallic luster, can conduct electricity and transfer heat. Because the distance between the layers is large, the binding force (Van der Waals force) is small, and the layers can slide, so the density of graphite is smaller than that of diamond, and the texture is soft and smooth.
In view of the high stability of the graphite material and the high specific capacity of silicon, a composite electrode of expanded graphite and silicon was prepared. A typical sandwich structure was formed between the silicon nanoparticles and the graphite sheet, which improved the electronic conduction characteristics of the material. Good performance (Carbon, 2014, 72: 38-46). On this basis, the expanded graphite was acidified and a silane coupling agent was added to achieve uniform dispersion of the silicon nanoparticles between the graphite sheet layers. The prepared composite electrode sheet was still close to 450 times at a current density of 0.4 A / g Specific capacity of 800 mAh / g.
Source: Encyclopedia, Shanxi Institute of Coal Chemistry
CNC Machining Automotive Parts
Cnc Machining Automotive Parts,Cnc Machined Car Parts,Billet Aluminum Auto Parts,Aluminum Disc Brakes
Yihua Precision Machining Co, Ltd. , https://www.yhmachining.com
